Material Science – Organic Matrix
Program Line 1
Bone is a hierarchically organized composite material with remarkable mechanical properties. The aim of the PL Material Science – Organic Matrix is to further advance our understanding of the contribution of the mineral and especially organic matrix quality in the determination of bone’s resistance to fracture, and to use the resulting data to develop spectroscopic technology as a clinical, diagnostic aid for musculoskeletal diseases.
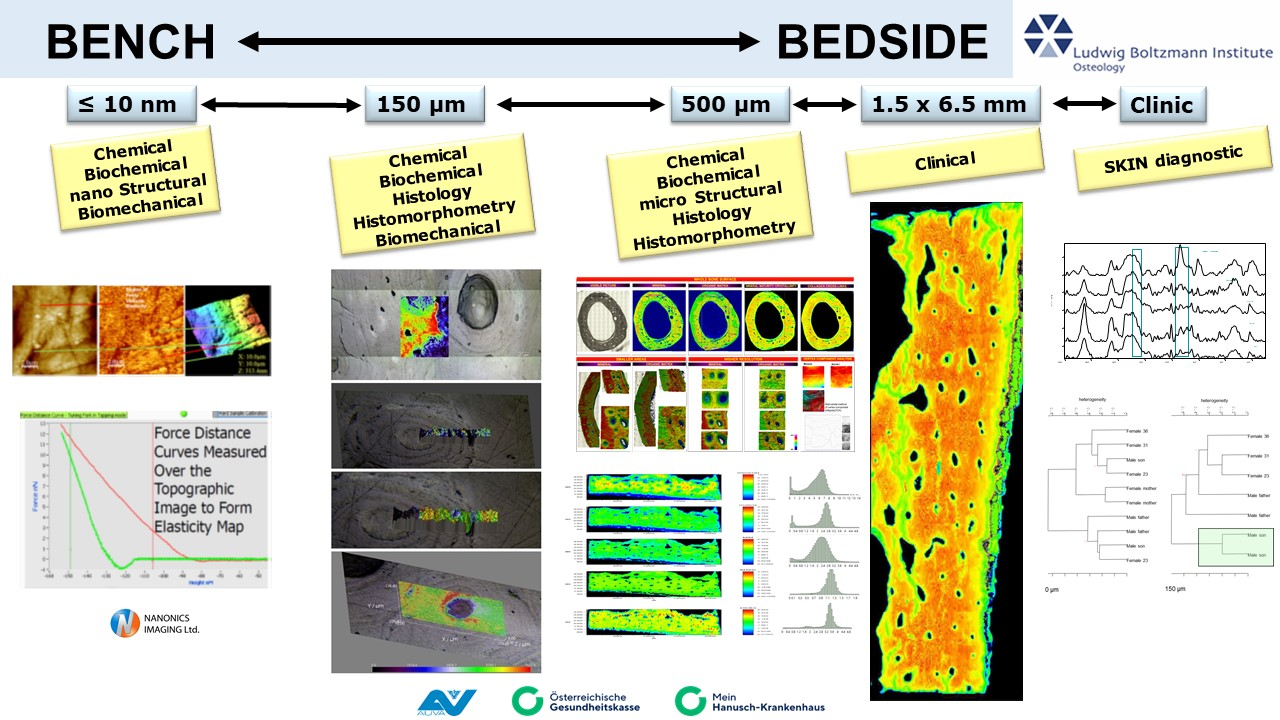
To achieve this, we use Fourier transform infrared imaging (FTIRI) and Raman spectroscopic (RS) techniques to analyze healthy and mechanically compromised bone, as well as soft tissues, at all hierarchical levels, with the ultimate goal of connecting bench with bedside.
Why Bone Quality?
In the clinic, prediction of fracture risk is based on the evaluation of clinical risk factors together with bone mineral density (BMD) measured by DXA (dual x-ray absorptiometry). Yet, it is widely accepted that BMD is not the singular factor that determines bone fragility. Bone quality includes the structural and material properties of bone. When considering material properties, it should be kept in mind that organic matrix chemistry / biochemistry, and mineral chemistry are dependent on turnover rates, but also factors other than bone turnover, thus both patient- and tissue-age within the same patient should be considered.
Underlying principle of FTIR Imaging & Raman Spectroscopy
Bone consists of mineral, organic matrix and water. The quantity and quality of all three components determine its mechanical properties. To the best of our knowledge, vibrational spectroscopic techniques such as FTIRI and RS are the only ones capable of providing simultaneous quantitative and qualitative information on all three bone components.
The physical principle underlying both FTIRI and RS techniques is the transition between vibrational energy states of molecules Each molecule has its own unique vibrational features. Additionally, the neighboring molecular environment influences these features, thus affording additional information on the “molecular neighborhood”. In the following we present the parameters routinely assessed by vibrational spectroscopic analysis (for details see Paschalis et al. or Gamsjaeger et al.)
Mineral to Matrix ratio
This is a measure of bone density that uniquely includes the amount of organic matrix in the volume of analysis. It strongly correlates with ash weight measurements by thermogravimetric analysis. The importance of including the organic matrix content in quantitative considerations is highlighted in experiments that utilize tensile testing of gradually decalcified bovine bone tissue, the results of which suggest that the mineral offers mainly compressive, while the collagen tensile strength. This ratio is directly proportional to bending stiffness and failure moment, and a superior predictor of bone-bending stiffness compared to BMD alone.
Mineral maturity/crystallinity
Bone mineral consists of poorly crystalline, highly substituted, apatite crystallites. Although pure apatites consist of calcium (Ca2+, phosphate (PO43-) and hydroxyl (OH–) ions, biological ones include various crystal lattice substitutions such as magnesium (Mg2+), potassium (K+), and / or sodium (Na+) for Ca2+, acidic phosphate (HPO42-) for PO4, and carbonate (CO32-), which can actually substitute for either OH– (type A), or PO43- (type B), or be loosely bound on the crystal surface (labile). Additionally, crystal vacancies may be present in the crystallites. The type and extent of these substitutions and vacancies modulates the crystal solubility as well as the size and shape.
Carbonate content
One of the most abundant substitutions in biological apatite crystals is carbonate, which may be in the form of Type A, Type B, or labile. In healthy bone, the average carbonate content is ~ 6 wt%. The extent of carbonate substitution has been reported to be altered in bone susceptible to fragility fractures in both animal models, and humans.
Relative tissue water content/ nanoporosity
Tissue water content is an important contributor to the determination of bone strength (especially bone toughness). Both FTIRI and RS can measure this content both directly and indirectly.
Collagen cross-links
The most striking feature of type I collagen in mineralized tissues is its cross-linking chemistry and molecular packing structure, responsible for tensile strength and viscoelasticity of fibrillar matrices. We have developed spectroscopic outcomes that correlate with biochemically determined enzymatic collagen cross-links (pyridinoline, deoxypyridinoline, and divalent) for FTIR, and RS (pyridinoline).
Glycosaminoglycan content
Proteoglycans are non-collagenous constituents of the extracellular matrix, present in both cartilage and bone among many other tissues. They are characterized by the presence of one or more glycosaminoglycan polymers attached to a protein core. In bone, they realize multiple roles including the organic matrix assembly, the modulation of both organic matrix mineralization and remodeling rates, as well as preventing mineralization of the perilacunar matrix around the osteocyte lacunae, and the canaliculi in compact lamellar bone, safeguarding the unhindered interstitial fluid movement.
Lipid content
Lipids have been identified in the published literature as nucleators of collagen fiber mineralization, and part of the matrix vesicles membranes. Moreover, oxidized lipids serve as a substratum for AGEs (advanced glycation endproducts) formation and accumulation.
Acidic Phosphate
Acid phosphate substitution into the apatite crystal lattice has been shown to be inversely proportional to crystallinity, thus a key determinant of mineralized tissues mechanical properties and their response to treatment.
Non-enzymatic cross-links
Non-enzymatic collagen cross-links (advanced glycation endproducts; AGEs) are an assorted group of compounds, formed through the non-enzymatic glycation or glycoxidation of proteins, lipids, and nucleic acids with the most-investigated being carboxymethyl-lysine (CML) and pentosidine. AGEs formation and accumulation in mineralized tissues results in more brittle bone.
Glucose content
Bone modulates systemic glucose homeostasis, mainly via osteocalcin and insulin, although additional mechanisms are suspected. Recent data suggest that cellular glucose metabolism in osteoblasts, via hypoxia signaling in bone, influences whole-body glucose homeostasis.
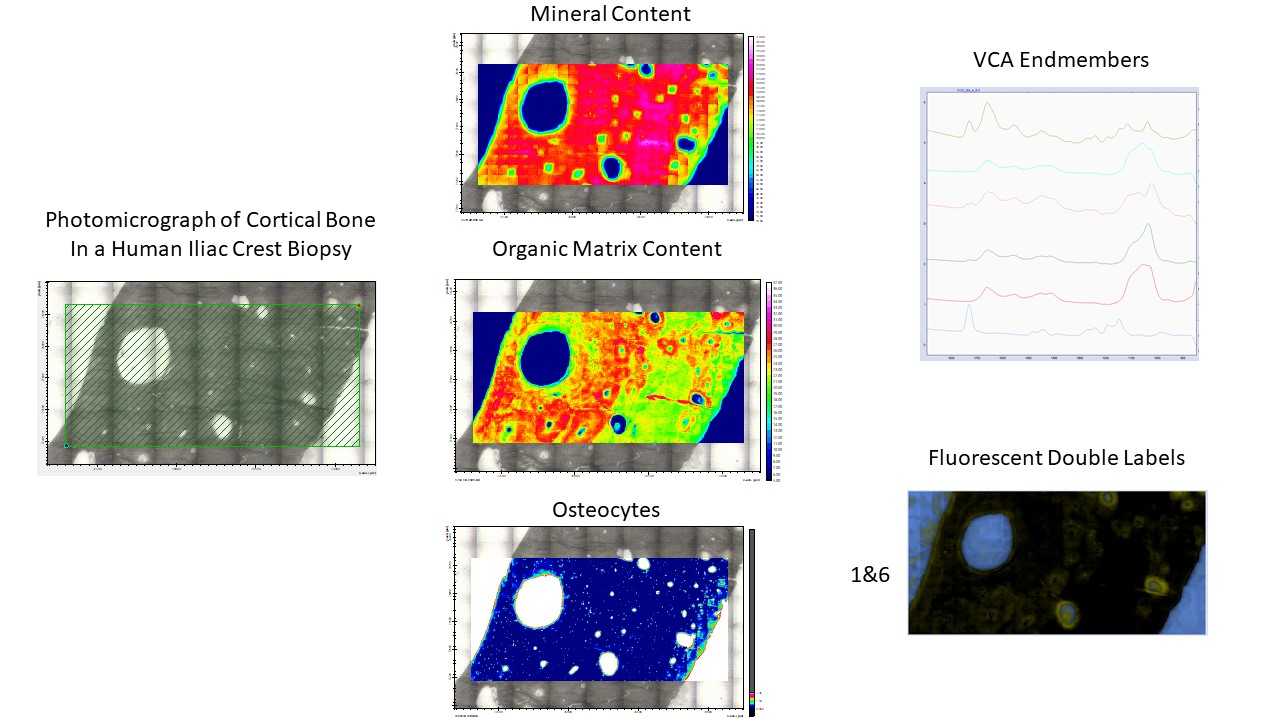
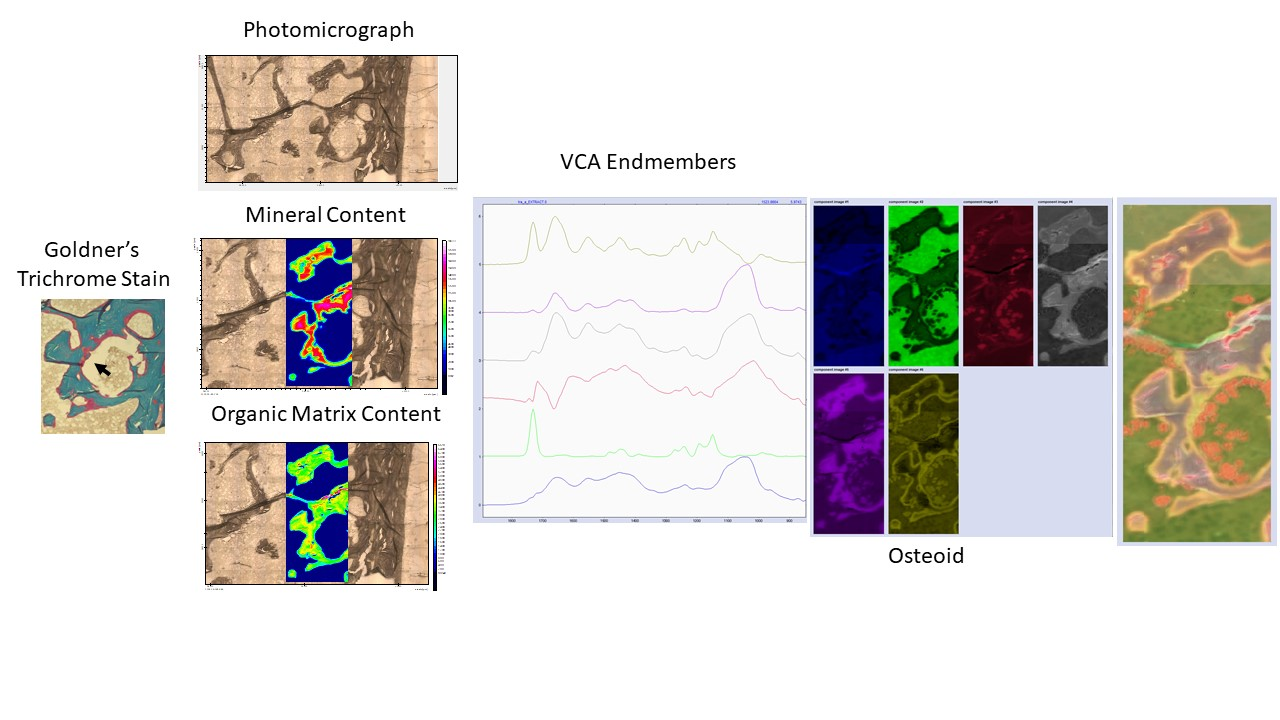
Chemometrics&Multivariate Imaging
Our upgraded FTIRI instrument allows the analysis of the whole bone biopsy surface. This results in spectroscopic images consisting of 20 – 100 million individual spectra. To handle such large datasets, we employ non-supervised methods such as VCA analysis amongst others to decipher the compositional information included in these images. This allows not only the calculation of mineral and organic matrix content, and osteocytes, but also the identification of fluorescent double labels and the description of osteoid.